When Laura Holmes Haddad was diagnosed with Stage IV inflammatory breast cancer, she felt like she needed a miracle. She shared her experience of receiving lifesaving treatment from a clinical trial and what led her to start advocating for fellow cancer patients.
Laura Holmes Haddad had always seen herself as a healthy person — she practiced yoga, naturally birthed both of her children, and was feeling good in her mid-30s.
“I always ate all my broccoli,” joked Holmes Haddad, now 48.
But six months after the birth of her son Roman, her second child, she felt something was wrong.
“I felt fatigued, I felt a lot of pain in my left arm and my upper chest — I thought I pulled a muscle,” Holmes Haddad said. “It also turned out I had a low-grade fever, which I didn’t even realize. I just didn’t feel like myself.”
After being misdiagnosed with mastitis, Holmes Haddad met with a breast oncologist who discovered an 11-centimeter tumor in her left breast.
“It was just before Thanksgiving 2012 that I learned it had spread and that it was very aggressive,” said Holmes Haddad, adding that the inflammatory breast cancer she was diagnosed with is found in only about 5,000 women each year in the United States.
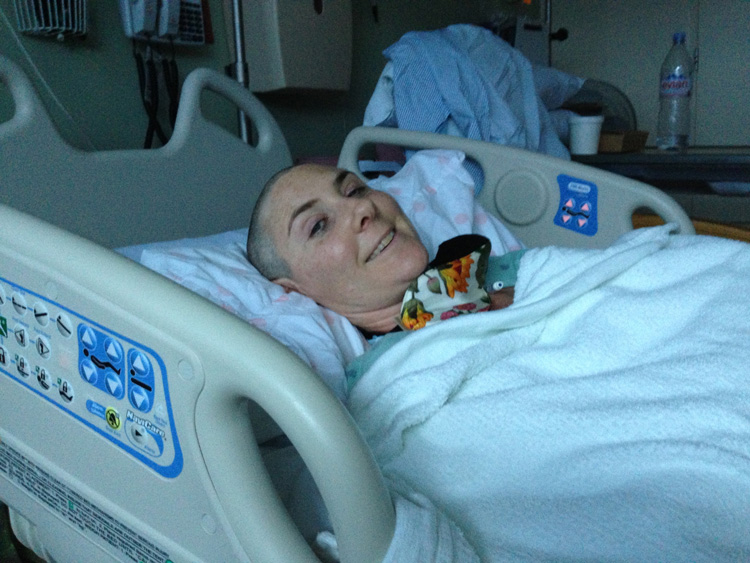
Treatment
Her care team originally set her on a course of adriamycin and cyclophosphamide (AC) chemotherapy to shrink the tumor to operable size, but it continued to grow throughout the treatment.
Holmes Haddad started meeting with doctors at UC San Francisco’s comprehensive cancer center — which puts a heavy focus on research and experimental therapies — to determine the best course of action. Her new oncologist, Dr. Mark Moasser, ordered a biopsy and genomic testing of the tumor, and found it was breast cancer gene 2 (BRCA2) positive and human epidermal growth factor 2 (HER2) negative.
“He [Dr. Moasser] said, ‘The only hope at this point is that you can get a clinical trial drug based on your genetic markers,’” Holmes Haddad said. “At the time, this precision medicine approach was very new in breast cancer, and he thought it could shrink this tumor.”
They found a clinical trial offering a treatment that could work at City of Hope in Los Angeles, however, there were a couple of problems.
First of all, Holmes Haddad lived in California’s Bay Area, about 350 miles away from L.A., and the trial would require her to come to City of Hope on a weekly basis. The test was also intended for ovarian cancer — not breast cancer — patients. And by the time she heard about the trial, recruitment and registration for it had closed.
Facing long odds to even get into the trial — let alone follow through with it — Holmes Haddad asked Dr. Moasser about what she should do.
“I had to ask him, ‘If I were your daughter, what would you have me do?’” she said. “And he said, ‘I would want you to go to the trial at City of Hope.’”
Despite being rejected five times in the first month she tried applying, Holmes Haddad was eventually accepted into the City of Hope trial on a compassionate use basis and began receiving the experimental treatment.

No evidence of disease
Traveling to Los Angeles every week for nearly six months to receive treatment caused Holmes Haddad and her husband Munir to rack up considerable expenses.
“Plane tickets, food, parking, rental cars, plus childcare for my kids at home, my family and I incurred all the costs,” Holmes Haddad said.
The costs and time commitment made the trial a major gamble, but it turned out to be worth it. The oral PARP inhibitor she was taking effectively reduced the tumor to 7 centimeters in size, the maximum allowable size for surgery.
Holmes Haddad’s care team sprang into action, performing a bilateral non-skin-sparing mastectomy and a salpingo-oophorectomy. They also removed 19 lymph nodes from under her arms.
She stayed on the clinical trial drug for almost two years after the procedure, but came off of it in May 2015 and was declared cancer-free.
“When you’re Stage IV, you’re never in remission — you’re in what’s called no evidence of disease, or NED,” Holmes Haddad said. “So, I’ve remained NED since May 2015.”
Where you live cannot be the determining factor in what kind of cancer care you receive.
Laura Holmes Haddad
Making lifesaving treatments accessible
Since surviving her own battle with cancer, Holmes Haddad has chronicled her experiences for and advocated on behalf of other patients. Already a published food and wine author, in 2016 she authored the book “This Is Cancer: Everything You Need to Know, from the Waiting Room to the Bedroom.”
She appropriately credits clinical trials with saving her life, and recommends all others who are eligible to pursue them — and not just for their own benefit.
“Your reaction to the experimental treatment, positive or negative, helps other patients down the line,” Holmes Haddad said.
While singing the praises of clinical trials, Holmes Haddad acknowledges the nation’s current system is in need of improvement. Her primary complaint is that most people don’t have the means to travel long distances to access these cures that are not yet widely available.
“It’s 2023 and unfortunately, I still think luck plays a big part in who can access precision medicine treatments in oncology,” Holmes Haddad said. “That has to change. Where you live cannot be the determining factor in what kind of cancer care you receive.
“Everyone has to work together to make these clinical trials and treatments more accessible, and also to make patients more comfortable with them.”

