Even with multiple SARS-CoV-2 vaccines now available, public health experts recognize the continuing need for rapid and reliable diagnostic testing.
Whether for triage, sports arenas, travel, or workplace testing programs, robust testing capabilities remain critical to opening society. Consequently, health authorities are dedicating significant resources to expand the available ecosystem of diagnostic testing.
The historical gold-standard tests for COVID-19 are polymerase chain reaction (PCR) molecular tests, which detect the genetic material of the virus. The best performing (detect low levels of virus) tests can be processed by a central lab, with options also available at the point-of-care (POC). These results vary in turn-around time, with central lab results often taking several days, or longer, when demand is high.
Rapid tests, in contrast, are typically antigen tests that look for viral proteins. These POC tests provide results in 15-20 minutes, with less complex workflows than PCR. However, they are generally perceived as less accurate because they typically require more virus to be present in the sample before they record a positive result — thus leading to more frequent false negatives, especially among people who don’t have symptoms. Strong need exists for antigen POC platforms that mimic PCR performance, but with typical antigen cost and workflows. Qorvo Biotechnologies is attempting to address this market gap.
This spring, the National Institutes of Health (NIH) through the Rapid Acceleration of Diagnostics (RADxSM) initiative, awarded a $24.4 million contract with funds from the biological advanced research and development authority, or BARDA, to advance the production and market launch of the Qorvo Biotechnologies Omnia™ test platform and SARS-CoV-2 antigen test. This recently received Emergency Use Authorization (EUA) from the Food and Drug Administration.
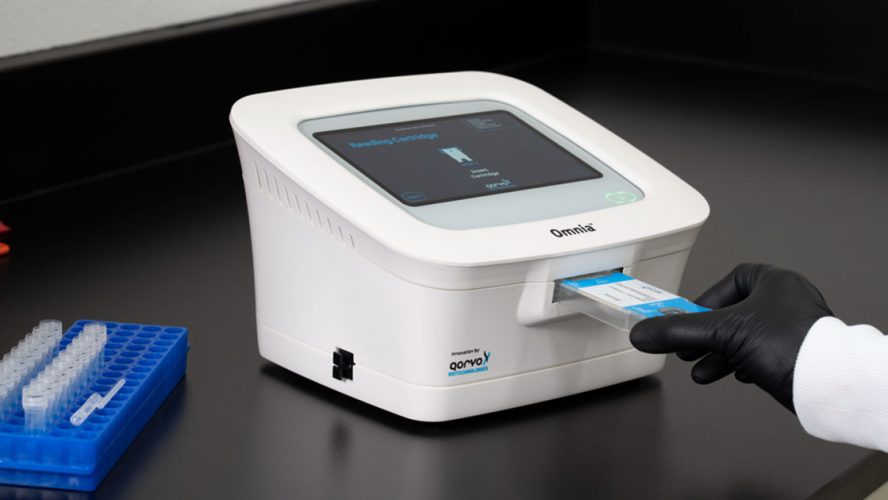
How does it work? Unlike traditional diagnostic approaches that rely on optical or fluorescence detection, the Qorvo Omnia system uses high-frequency Bulk Acoustic Wave (BAW) sensors — which are typically used in cellphones and Wi‑Fi routers — to detect the presence of COVID-19 antigens from an anterior nasal swab. A small cartridge containing the BAW sensor is used to measure a reaction between the biochemical coating on the BAW sensor surface and the sample being tested. As mass is added to the surface, a change in frequency occurs that can be converted to a known concentration.
The BAW sensor’s extremely high frequency (it resonates at 3 GHz, or 3 billion cycles per second) enables a low limit of detection (LOD) and is 100 percent specific, critical in low prevalence settings to minimize false positives.
“This new approach to testing has the potential to transform disease detection,” said Fred S. Apple, Ph.D., a member of Qorvo Biotechnologies’ advisory board, co-medical director of the Toxicology Laboratory at Hennepin Healthcare and Hennepin County Medical Center and professor of Laboratory Medicine & Pathology at the University of Minnesota. “The Qorvo Omnia system is focused on producing results comparable to PCR testing with the ability to be deployed for a wide range of rapid point-of-care diagnostic tests — including tests for the next deadly pathogen to cause an outbreak.”
With its combination of high sensitivity, specificity, and speed, BAW is well positioned to meet the ongoing need for rapid diagnostic testing in high-volume settings, helping American society return to normalcy.
*This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.