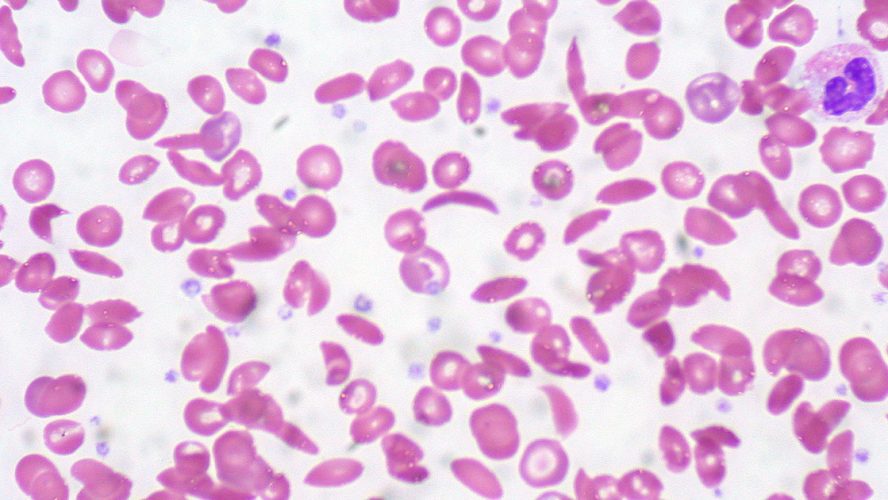
The Medical University of South Carolina (MUSC) Children’s Health has been a national leader in sickle cell disease treatment and research. We talked to Drs. Sherron M. Jackson and Shayla Marie Bergmann about how their work benefits the region and the country.
What is the history of sickle cell patient care at MUSC Children’s Health?
Sherron Jackson: The sickle cell program began in 1985 as a newborn screening clinic and boasts over 30 years of patient care and clinical research. The first in the state, the MUSC program was the catalyst for the 1987 statewide newborn screening mandate.
How is MUSC improving pediatric sickle cell care in the region?
Shayla Bergmann: MUSC’s understanding of this complex blood disorder supports children and adults up to age 25 throughout South Carolina. Successful treatment of our large population of patients allowed for participation in national clinical trials, leading to the development of national standards of sickle cell care.
Tell us about your therapies management and pain management programs.
SJ: The major therapy to prevent bone pain episodes is hydroxyurea. Each patient has a written plan for managing pain at home (oral medications) and in the emergency department, clinic, or hospital (intravenous). Recent FDA-approved agents that modify sickle hemoglobin are provided for eligible patients in our clinic.
Dr. Jackson, what are your particular interests in treating sickle cell?
SJ: I meet the babies at birth and work with families to educate them about the disease, and then create the best care plan based on our proven therapies and pain management programs.
Dr. Bergmann, what are your areas of interest in treating sickle cell?
SB: I am particularly interested in the access to clinical trials we provide our patients. With 10 ongoing trials and six on deck, I am very excited about our research in the areas of molecular modification, gene therapy, CRISPR, and bone marrow transplant (BMT).
What research projects has MUSC participated in?
SJ: MUSC was instrumental in the NIH-funded trials in sickle cell children including stroke prevention using transcranial doppler (STOP), hydroxyurea (Baby HUG), and the Bluebird trial using gene therapy to cure the disease.
What is the future of sickle cell treatment at MUSC Children’s Health?
SB: The future is busy and bright. Aggressive research creates new and improved treatments, providing families with more options. There are more treatment possibilities through CRISPR, new genetic therapies and molecular modification, and improvements in existing therapy and pain management options. Additionally, telehealth improves access to our experts through technology, allowing patients to better maintain care plans and make adjustments in real time.